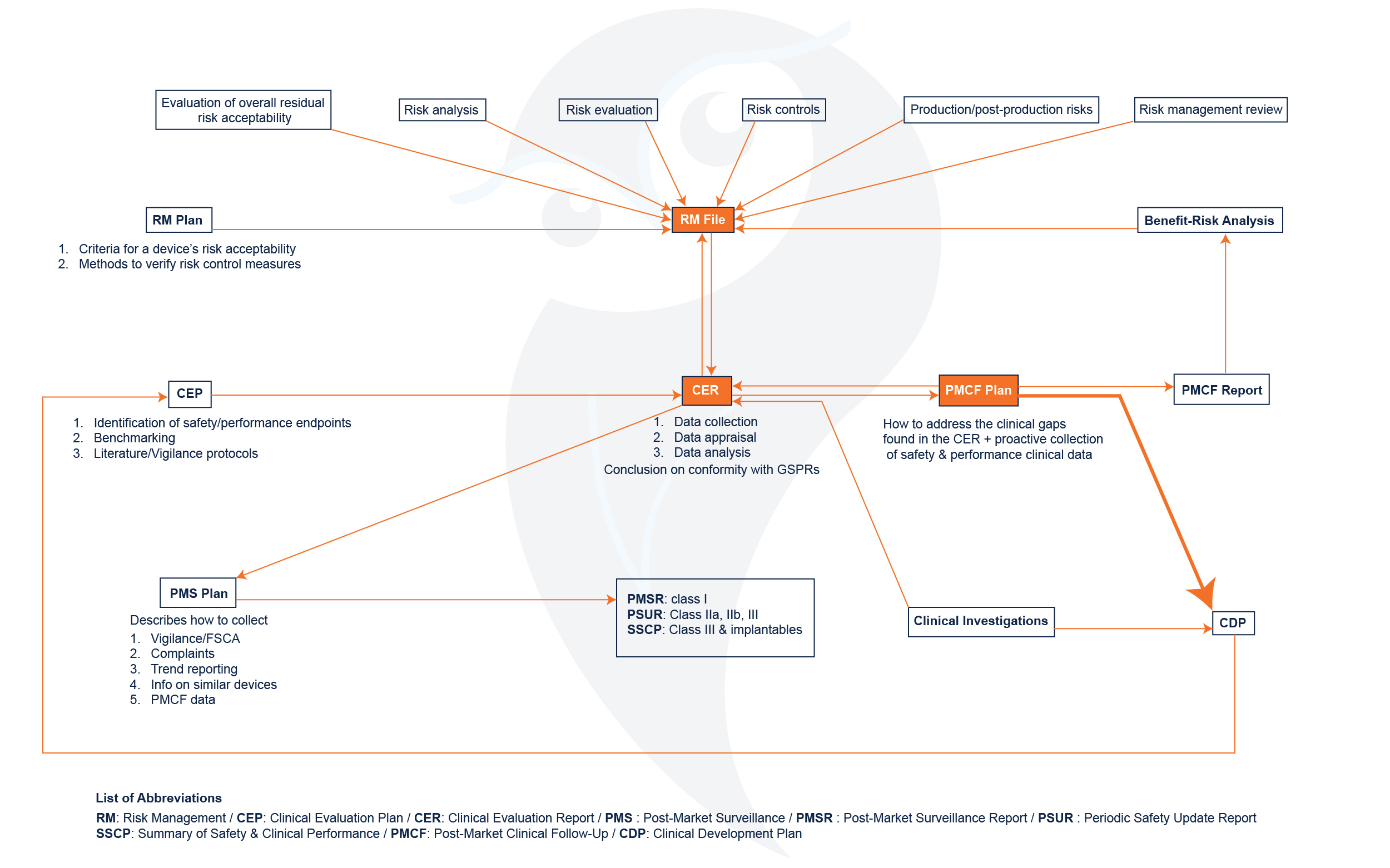
Achieving and maintaining conformity with MDR requirements can be a strenuous process.
In Evnia, our full-value-chain services are supported by a growing, cross-functional team of medical writers, medical doctors, engineers for usability, risk, and validation, biostatisticians, biocompatibility experts, and regulatory affairs executives. Our in-house human resources enable the development and implementation of holistic approaches covering the entire lifetime of medical products.
We offer a cluster of interconnected services from the early stages of a medical device’s lifecycle until its post-market adulthood supporting healthcare innovation and promotion of patient safety in the fields of:
📌 Due Diligence
📌 Regulatory Strategy
📌Clinical Development Strategy
📌 Post-Market Surveillance
📌 Real World Evidence
📌 Market Access and Reimbursement
📌 EU and UK Representation Services (Authorised Representative & UKCA/UKRP)
Reach out for a preliminary discussion to identify solutions tailored to your needs.