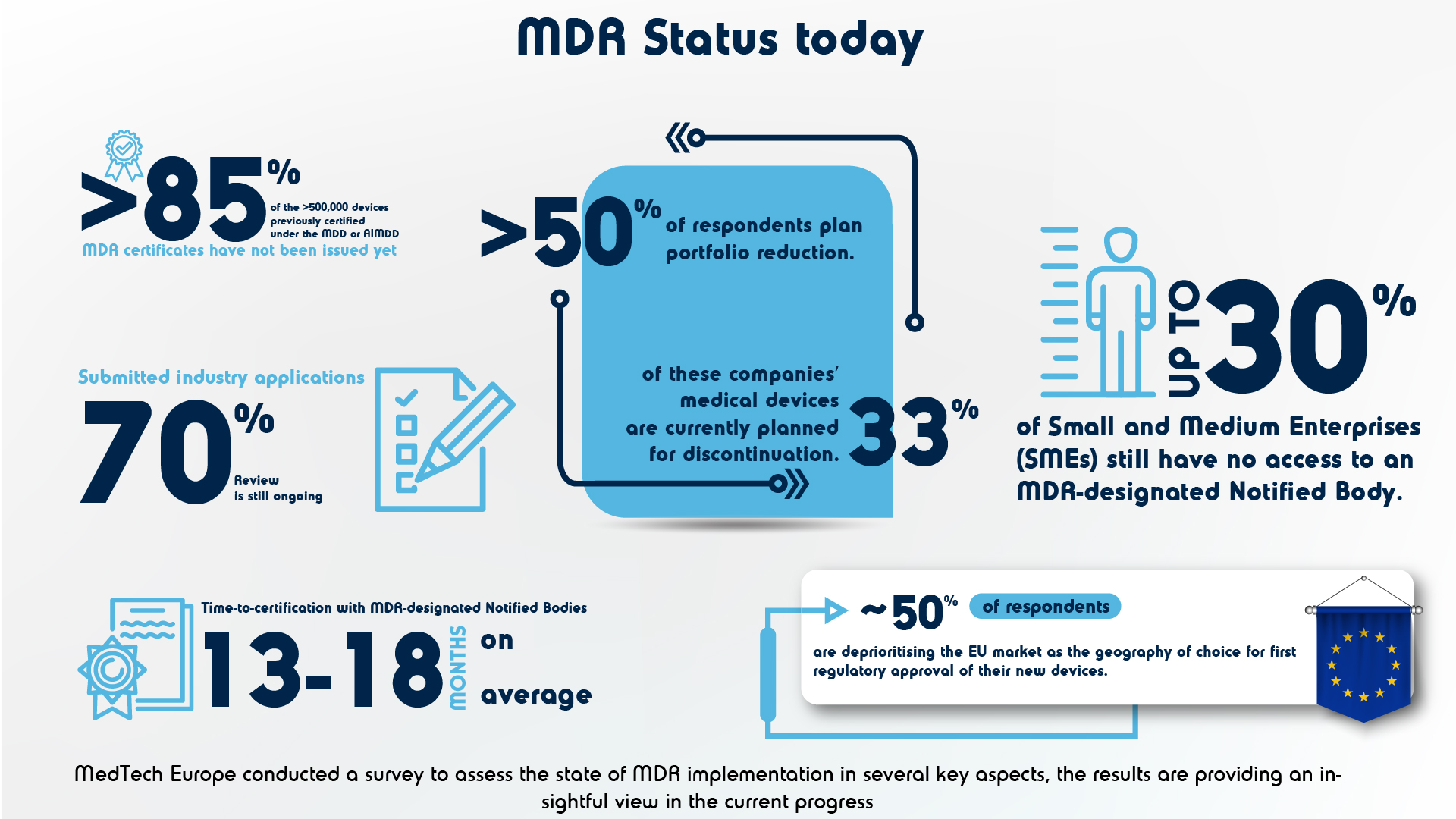
MedTech Europe has published a survey report to analyse the availability of medical devices and other key aspects in relation to the ongoing MDR implementation. The survey clearly reflects a challenging and growing bottleneck, especially around SMEs, and highlights the need for immediate actions to ensure the continued availability of medical devices in Europe.
Among the most prevalent challenges lie
📌 The transitioning of AIMDD/ MDD certificates to MDR: >500,000 devices certified under the old directives have yet to be converted to MDR, while 54% of the respondents plan portfolio reductions.
📌Progress of Notified Bodies in treating applications submitted for MDR certification: >85% of the products previously certified under the old directives
📌Notified Body capacity limitations and assessment timelines: >30% of smaller businesses lack access to MDR-designated notified bodies
📌 Lack/Delay of necessary guidelines / guidances / common specifications
Read the full report here