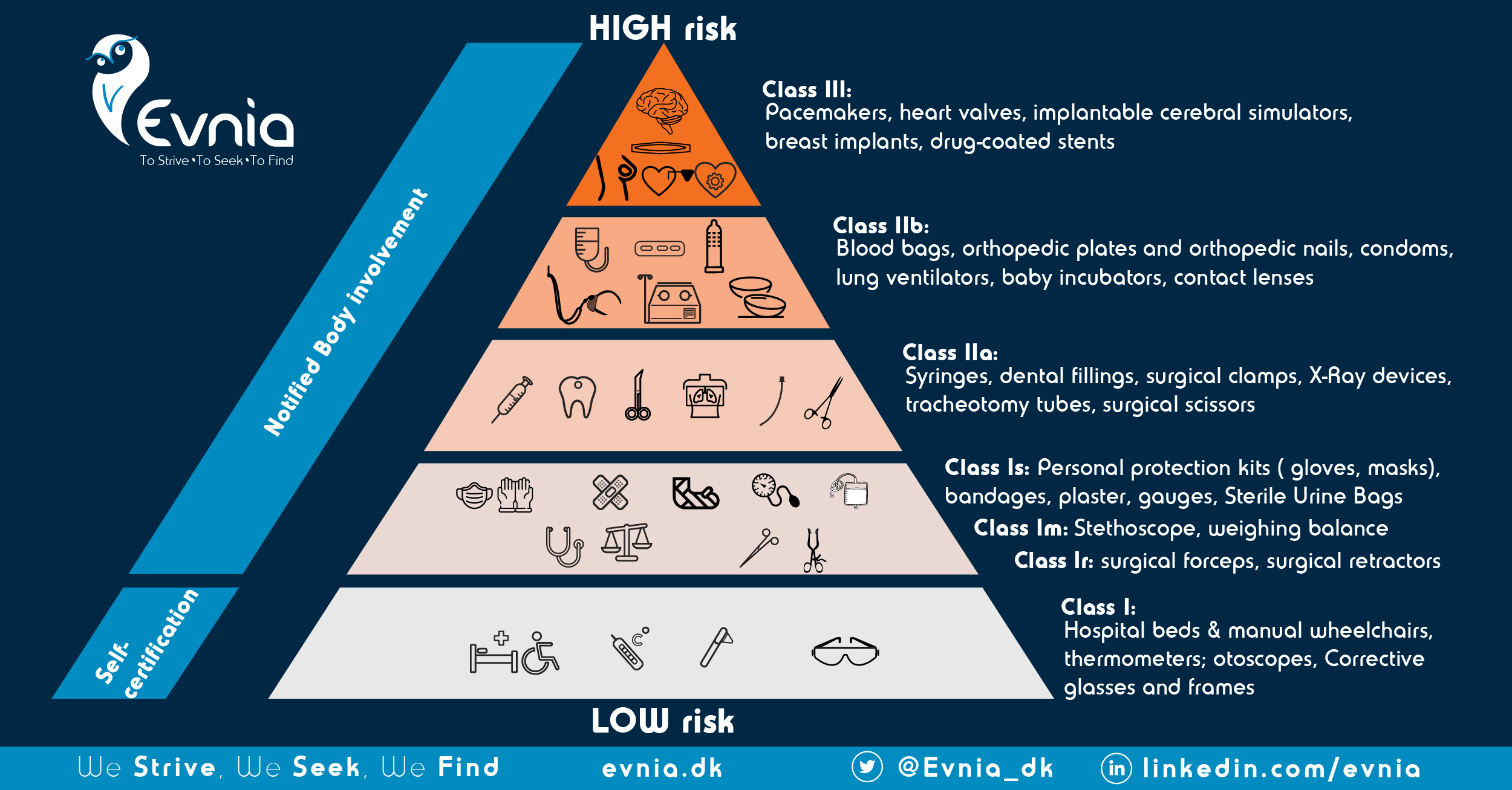
EU MDR is a risk-based Regulation. Unlike MDD, which did not include an explicit requirement to employ risk management activities, other than for software devices, Art. 10(2) explicitly states that Manufacturers should establish, document, implement and maintain a system for risk management (plan, analysis, and report; see how in Annex I; Chpt I), that will cover the full intended lifetime of the device. Practically, this entails alignment of literature-identified risks with PMS, PMCF activities but also with V&V including usability engineering studies.
Looking for guidance on how to include Risk Management at every level of your Quality System? Having trouble going from ALARP to ‘as low as possible’? Contact Evnia today for a tailored consultation!